Research
Synthesis of fluorinated carbohydrates
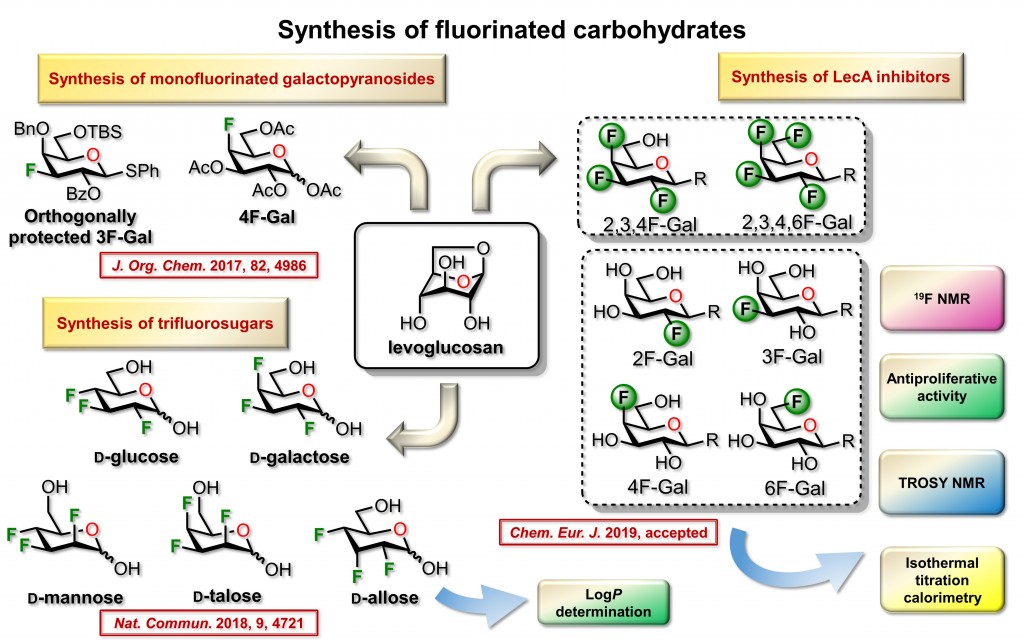
Our research group focus on the synthesis of fluorinated sugars. Carbohydrates bearing a fluorine atom in place of a hydroxyl group have found applications in biochemical investigations and radiolabeling. The replacement of hydroxyl groups with fluorine atoms to generate fluorine-substituted analogs of naturally occurring or biologically active organic compounds is extensively studied. The rationale to prepare such compounds arises from similarities between OH group and F atom in regard to polarity and isosteric relationship. Another important feature is the loss of hydrogen donating capacity for the F atom and the high C−F bond energy renders them resistant to metabolic transformation. Finally, the addition of a fluorine group can lead to greater lipophilicity, which in turn can increase bioavailability, tissue distribution and cell permeability.
The stereoselective synthesis and conformational analysis of polyfluorinated derivatives, and a fortiori multi-vicinal organofluorine isomers, remain a tedious challenge. Consequently, systematic biological investigations of heavily fluorinated carbohydrates have often been impeded by the weak efficiency of the multistep sequences used in de novo approaches. We used a Chiron approach for the preparation of heavily fluorinated sugars. The accessible variety of synthesized derivatives spans over seven distinct members of functionalized carbohydrates. More specifically, original polyfluorinated D-galactopyranoside, D-galacturonic acid methyl ester, D-fucoside and also trifluorinated D-glucose derivative, D-mannose, D-talose, and D-allose.
Our methodology included practical features that allowed operations on a large scale starting from inexpensive starting material. The proposed sequences enable a minimal usage of protection/deprotection cycles, allow an excellent regio- and stereocontrols; and avoid tedious purifications. We used 1,6-anhydro-β-D-glucopyranose (levoglucosan) as inexpensive starting material. This choice has been motivated since the 1,6-anhydro core prevents protection of both O-6 and anomeric positions and allows navigation on the pyran ring to install fluorine atoms using simple experimental protocols.
Synthesis of medically relevant carbohydrates
Our research program focus on the synthesis of medically relevant glycopeptides and glycoproteins.
Total synthesis of biologically active natural products
Our research group is also implicated in the preparation of natural products with interesting biological activities.